Background:
A subset of follicular lymphoma (FL) patients will experience a ‘high-risk defining event’ (HRDE) that portends poor survival due to early progression of disease or transformation to diffuse large B cell lymphoma (TFL). Progression of disease within 24 months (POD24) from front-line immunochemotherapy (ICT) identifies patients with a subsequent five-year OS of 50% compared to a reference group of 90% (Casulo et al. JCO 2015). Although a robust surrogate endpoint for OS in clinical trials (Casulo et al. Blood 2022), it remains to be established if the OS of patents is influenced by the nature of the HRDE. In this study, we examined whether characteristics and clinical trajectory of those with relapsed/progressive FL is the same as that of patients experiencing TFL.
Methods:
We conducted an international retrospective analysis of newly diagnosed FL patients requiring treatment across 14 academic centres between 2002 and 2021 in the era of rituximab and PET-CT staging. Patients were included with grade 1-3A FL requiring first-line (1L) treatment (ICT, rituximab monotherapy or radiotherapy). We excluded patients with untreated FL, de novo TFL and those with <24 months follow up provided they did not experience a HRDE. Baseline clinical, imaging and laboratory values, treatment details and outcomes were collected. We defined HRDE groups as persistent (relapse or progression) FL within 24 months of 1L treatment (FL24), early TFL (<24 months of 1L treatment) or late TFL (>24 months of 1L treatment). Patients not experiencing a HRDE formed the FL reference group. Landmark overall survival (OS) analysis was performed by Cox regression using risk status as a time-varying covariate for time of HRDE. For the FL reference group, landmark was defined at 24 months after 1L treatment.
Results:
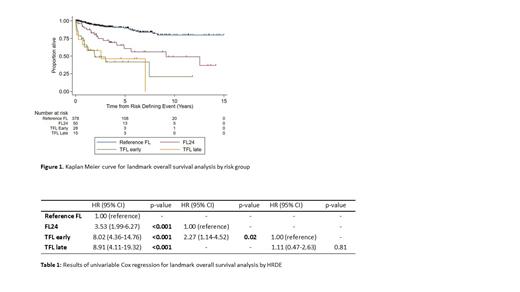
501 patients met the inclusion criteria and were categorised as: reference FL (n=378), FL24 (n=50), early TFL (n=28), late TFL (n=15). 24 patients who died within 24 months of 1L treatment without documentation of preceding HRDE and were omitted. Median follow up for patients alive beyond 24 months was 5.1 years. Median age at diagnosis was 60 years, 91% had ECOG performance status 0-1 and 38% were high-risk (>3) FLIPI. 1L chemotherapy regimens included: bendamustine (59%), CHOP (24%), CVP (8%), other (9%); a majority received rituximab (80%) vs obinutuzumab (20%). Maintenance therapy was used in 54% of pts.
Expectedly, those experiencing HRDE had an inferior OS compared to the reference group (HR 5.23, p<0.001)(Figure 1). However, among HRDE group, median OS significantly differed: early TFL patients (3.0 yrs) and FL24 (12.6yrs)(HR 2.27, p=0.02) (Table 1). There was no difference in OS after HRDE between early TFL and late TFL ( p=0.81). Biopsy was performed in 84% of TFL cases with no difference in OS between biopsy-proven vs clinically suspected cases ( p=0.23). Multivariate OS analysis assessing HRDE and treatment demonstrated no significant interaction between HRDE group and 1L chemotherapy ( p=0.52), monoclonal antibody ( p=0.29) or maintenance therapy ( p=0.27).
Several established prognostic features at baseline were enriched in patients who went on to experience HRDE including: ECOG >1 ( p<0.001), raised LDH ( p<0.001), advanced-stage disease (( p<0.011) and high risk FLIPI ( p=0.005). Baseline FDG-PET was available in 259 patients. Adverse PET metrics were enriched in only early TFL, and not FL24 or late TFL patients, including high SUV max( p=0.009), total metabolic tumor volume ( p=0.015) and total lesional glycolysis ( p=0.01).
Conclusions:
HRDEs after 1L treatment are associated with inferior OS in FL compared with standard-risk patients. Unlike POD24, this study demonstrates disparate clinical outcomes between patients experiencing early progression with persistent FL and those experiencing TFL. Adverse metabolic characteristics on baseline FDG-PET also suggest biological distinction between HRDE subsets. These findings have important implications for epidemiological studies, biomarker development and clinical trials aiming to improve outcomes in this underserved high-risk FL cohort.
Disclosures
Tobin:Roche: Honoraria. Cochrane:Imago BioSciences, Inc., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA: Research Funding; Beigene: Research Funding; Janssen-Cilag: Speakers Bureau. Trotman:Cellectar: Research Funding; Janssen: Research Funding; Roche: Research Funding; BeiGene: Research Funding; BMS: Research Funding. Talaulikar:Beigene: Honoraria; Amgen: Honoraria; Takeda: Honoraria; EUSA: Honoraria; CSL: Honoraria, Speakers Bureau; Janssen: Honoraria, Research Funding, Speakers Bureau; Roche: Honoraria, Research Funding, Speakers Bureau; Antengene: Honoraria. Shortt:Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding; Otsuka: Consultancy, Membership on an entity's Board of Directors or advisory committees; Mundipharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees. Cheah:Lilly: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Ascentage Pharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AstraZenecca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: TRAVEL, ACCOMMODATIONS, EXPENSES, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Research Funding; Dizal: Consultancy, Honoraria; Menarini: Consultancy, Honoraria; Genmab: Consultancy, Honoraria; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BeiGene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; TG therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; MSD: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Barraclough:Gilead: Consultancy, Honoraria; Roche: Honoraria. Ansell:Affirmed: Other: Contracted Research; Pfizer, Inc: Other: Contracted Research; ADC Therapeutics: Other: Contracted Research; Regeneron Pharmaceuticals Inc: Other: Contracted Research; Bristol-Myers Squibb: Other: Contracted Research; Seagen Inc: Other: Contracted Research; Takeda Pharmaceuticals USA Inc: Other: Contracted Research.